Medical Device Outsourcing Market Size to Reach USD 375.3 Billion by 2032 growing at 12.7% CAGR - Exclusive Report by Acumen Research and Consulting
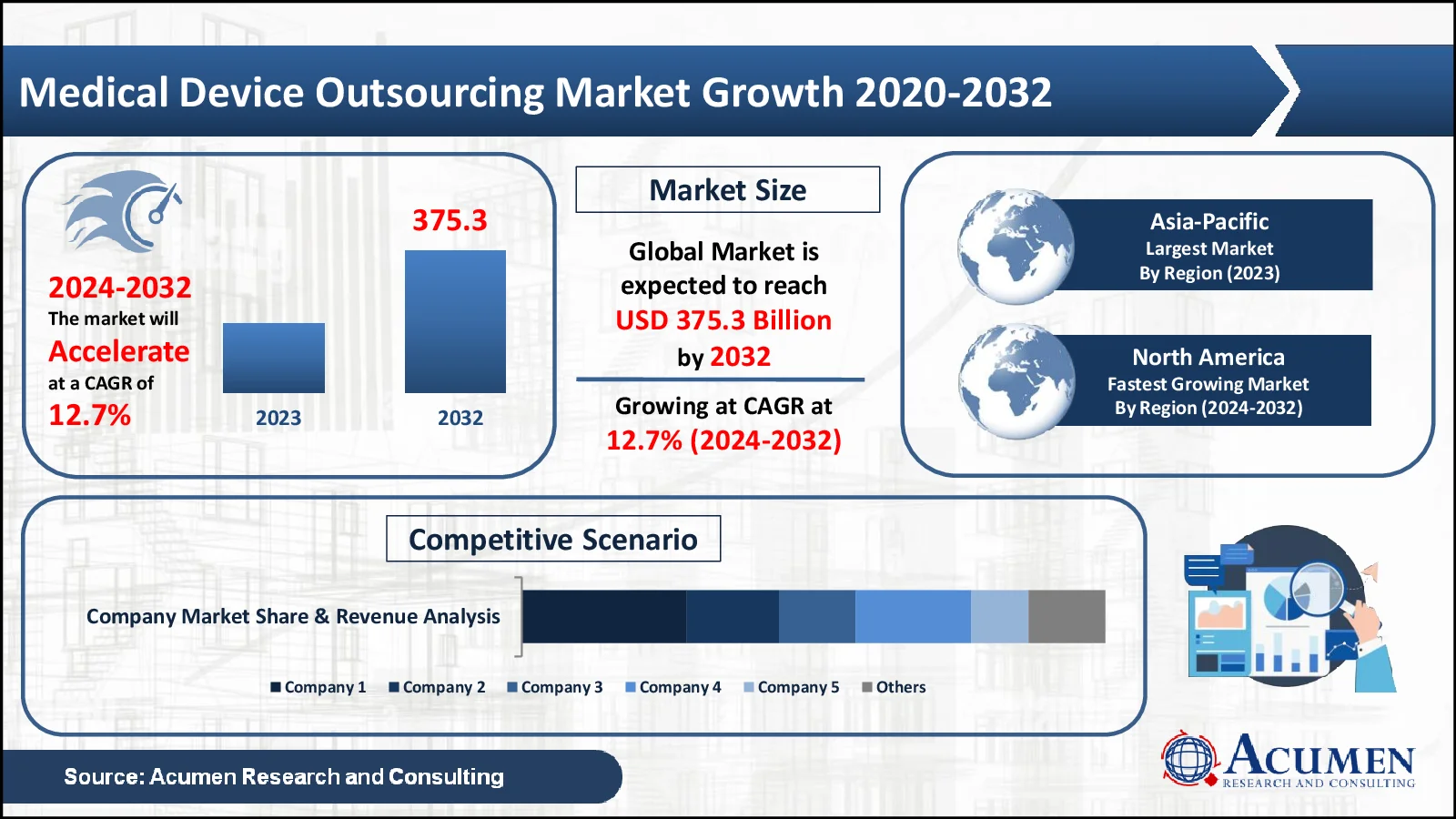
The Medical Device Outsourcing Market, estimated at USD 129.2 billion in 2023, is expected to exceed USD 375.3 billion by 2032, with a projected CAGR of 12.7%
The medical device outsourcing market is expanding significantly due to several key factors. Companies in this sector are increasingly opting for outsourcing to achieve cost efficiency and streamline operations. By delegating manufacturing and other functions to specialized third-party providers, medical device companies can reduce operational expenses associated with production, research and development, and regulatory compliance. These outsourcing partners bring advanced technologies and specialized expertise, which help in lowering costs and enhancing overall operational efficiency.
A major driver of this market is the complexity of regulatory requirements. Outsourcing partners often have deep knowledge of regulatory standards and help companies navigate these complexities, ensuring compliance with international regulations and facilitating faster market entry. Additionally, the rapid pace of technological advancements in medical devices necessitates continuous innovation. Outsourcing enables companies to leverage the latest technologies and stay competitive without heavy internal investment in research and development.
Despite the benefits, the market faces challenges such as intellectual property risks and quality control issues. However, ongoing improvements in outsourcing practices and strategic partnerships offer opportunities for growth and innovation. Overall, the Medical Device Outsourcing market presents a promising landscape for companies looking to enhance efficiency and remain competitive in the evolving healthcare sector.
Medical Device Outsourcing Market Statistics
- 2023 Milestone: The global medical device outsourcing market reached a significant value of USD 129.2 billion
- Growth Projection: The market is expected to grow at a robust compound annual growth rate (CAGR) of 12.7% from 2024 to 2032
- Regional Dominance: Asia-Pacific holds a commanding 41% share, making it the largest regional market
- Fastest-Growing Region: North America is growing at an exceptional rate of 13.4%, presenting lucrative opportunities for industry participants
- Key Segment: The contract manufacturing service category is a notable segment within the market
- Revenue Driver: The cardiology application has been instrumental in driving revenue growth
- Trend: Expanding into emerging markets is a significant trend shaping the industry
Request for a sample of this premium research report@ https://www.acumenresearchandconsulting.com/request-sample/1020
Medical Device Outsourcing Market Dynamics
Cost Efficiency Fuels the Medical Device Outsourcing Market
Cost efficiency is a major driver in the medical device outsourcing market. By outsourcing critical functions such as manufacturing, research and development (R&D), and regulatory compliance, medical device companies can substantially reduce operational costs. Outsourcing eliminates the need for significant capital investments in manufacturing facilities, specialized equipment, and skilled personnel. Outsourcing partners, who already possess established infrastructures and expertise, can deliver these services more cost-effectively. They benefit from economies of scale, serving multiple clients and spreading costs over a larger base, which enables them to offer competitive pricing. This approach allows medical device companies to reallocate their resources towards core activities like innovation and strategic growth, enhancing their overall efficiency and reducing overheads. Additionally, outsourcing accelerates time-to-market for new products by leveraging the streamlined processes of these specialized partners, ultimately contributing to a more agile and cost-effective operational model.
Technological Advancements Offers Significant Medical Device Outsourcing Market Opportunities
An emerging opportunity in the medical device outsourcing market lies in leveraging technological advancements. The rapid evolution of medical technologies presents significant potential for outsourcing companies to offer innovative solutions and services. As the medical device industry increasingly integrates sophisticated technologies such as advanced robotics, artificial intelligence, and digital health tools, there is a growing demand for outsourcing partners that can provide cutting-edge capabilities.
Outsourcing firms that invest in and adopt the latest technological advancements can offer enhanced services, such as improved product development, higher precision manufacturing, and advanced regulatory compliance solutions. For instance, incorporating AI into design and manufacturing processes can lead to more efficient and accurate production, while digital health technologies can enable real-time data analysis and improved patient outcomes.
Moreover, outsourcing partners can facilitate access to these technologies for companies that may not have the internal resources to develop or implement them. This allows medical device companies to stay competitive in a rapidly evolving market without making substantial internal investments. By partnering with firms that are at the forefront of technological innovation, companies can enhance their product offerings, speed up development cycles, and enter new markets with advanced solutions.
Medical Device Outsourcing Market Segmentation
The global market for medical device outsourcing is divided into 3 segments: Service, application, and regional markets
- Service: quality assurance, regulatory affairs services, product design and development services, product testing & sterilization services, product implementation services, product upgrade services, product maintenance services, and contract manufacturing
- Application: cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care, and others
- Regional industry: Europe, Latin America, Asia-Pacific, North America, and the Middle East & Africa
Medical Device Outsourcing Market Regional Outlook
The Asia-Pacific region stands as the largest market, characterized by its substantial growth potential and cost advantages. Countries such as China and India are emerging as key players in the outsourcing landscape due to their lower operational costs and expanding healthcare infrastructure. The increasing demand for medical devices and substantial investments in healthcare within these countries drive the growth of outsourcing services. The region's large pool of skilled professionals and its rapidly evolving healthcare sector contribute to its position as the largest market for medical device outsourcing.
Medical Device Outsourcing Market Players
Medical device outsourcing companies profiled in the report include Intertek Group plc, SGS SA, Medical Device Testing Services, Eurofins Scientific, Charles River Laboratories, Pace Analytical Services, Inc., North American Science Associates, LLC, Laboratory Corporation of America Holdings, WuXiAppTec, RJR Consulting, Inc., Sterigenics U.S., LLC (GTCR, LLC), and TÜV SÜD.
Click here to buy the Premium Market Research report https://www.acumenresearchandconsulting.com/buy-now/0/1020
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/1020
Mr. Richard Johnson
Acumen Research and Consulting
India: +918983225533
E-mail: [email protected]


