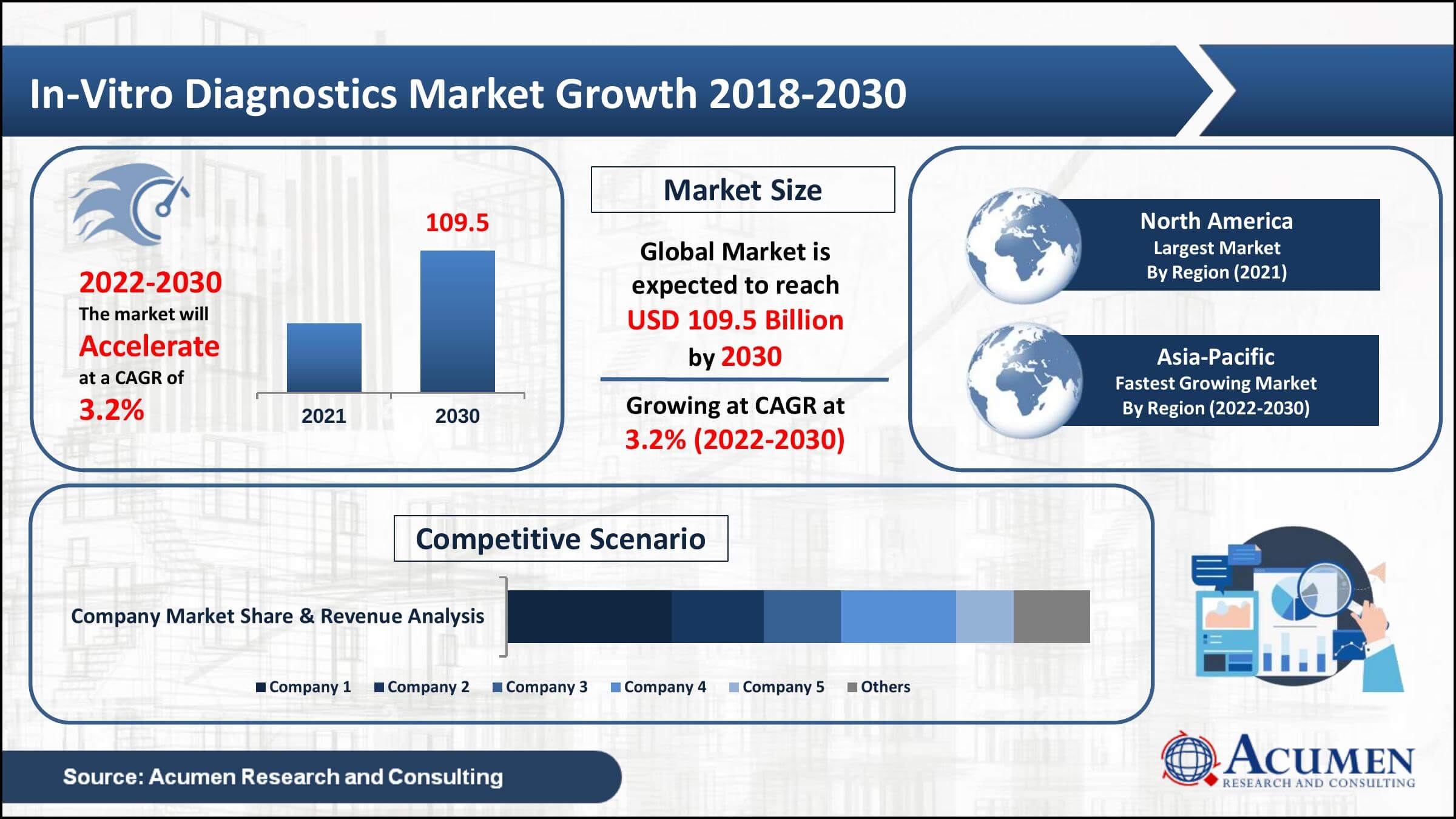
In-Vitro Diagnostics Market Size is Expected to Reach at USD 109.5 Billion by 2030, Registering a CAGR of 3.2%, Owing to Increasing Incidence of Chronic Diseases
The Global In-Vitro Diagnostics Market Size is predicted to reach USD 109.5 Billion by 2030 from USD 83.3 Billion in 2021, at a CAGR of 3.2% between 2022 and 2030, as per the Acumen Research and Consulting
The In-Vitro Diagnostics (IVD) market is a rapidly growing field, driven by advances in technology, an aging population, and increasing rates of chronic diseases. The market is expected to continue to grow in the coming years, as new diagnostic tests and technologies are developed and adopted. Some key trends in the IVD market include the shift towards point-of-care testing, the use of biomarkers and personalized medicine, and the increasing use of digital technologies in diagnostics. Additionally, the COVID-19 pandemic has accelerated the adoption of telemedicine and home-based testing, further driving growth in the IVD market.
In-Vitro Diagnostics Market Statistics
- Global in-vitro diagnostics market revenue collected USD 83.3 Billion in 2021, with a 3.2% CAGR between 2022 and 2030
- North America in-vitro diagnostics market value gathered more than USD 32 Billion in 2021
- According to research, 900 million tests are performed in the United Kingdom each year
- Among applications, the infectious disease category capture over 60% of total market share in 2021
- Increasing adoption of home-based diagnostic testing, fuels the in-vitro diagnostics market size
Request for a sample of this premium research report@ https://www.acumenresearchandconsulting.com/request-sample/988
In-Vitro Diagnostics Market Trends
In-vitro diagnostics (IVD) involves the investigation of biological samples, such as blood or urine, outside of a living body. This can involve disease diagnostic tests, chronic condition monitoring, and medicine effectiveness testing. IVD can be performed in a clinical setting, such as a hospital or doctor's office, or at home using a self-test kit. The major drivers for the IVD market include an aging population, the increasing prevalence of chronic diseases, and advancements in technology leading to more accurate and efficient diagnostic tests. However, there are also some restraints to the market, such as reimbursement issues and regulatory hurdles.
Opportunities for growth in the IVD market include the development of point-of-care testing, which allows for quicker and more convenient testing, as well as the use of biomarkers for personalized medicine. Additionally, there is potential for growth in emerging markets, as well as the use of IVD in developing countries for the detection of infectious diseases.
COVID-19 Pandemic Has Accelerated the IVD Market Growth
The COVID-19 pandemic has had a significant impact on the IVD market, accelerating its growth in several ways. One of the most significant drivers of growth has been the increased demand for diagnostic tests for COVID-19, both in terms of PCR tests for active infection and antibody tests for past infection. This has led to a rapid expansion in the production and availability of these tests, as well as the development of new, more efficient testing methods.
The pandemic has also led to an increase in the demand for remote monitoring and self-testing, as people try to avoid going to hospitals or clinics. This has led to a growth in the market for home-use IVD tests, as well as the development of new technologies such as telemedicine and digital health.
Additionally, the pandemic has accelerated the adoption of point-of-care testing, which allows for quicker and more convenient testing, as well as the use of biomarkers for personalized medicine. Moreover, the pandemic has also highlighted the importance of decentralization of healthcare services and the need for more efficient and cost-effective diagnostic tools, which are driving the IVD market.
In-Vitro Diagnostics Market Segmentation
Acumen Research and Consulting has segmented the global in-vitro diagnostics market by technology, application, product, and end-use.
- By technology, the industry is categorized into clinical chemistry, microbiology, molecular diagnostics, immunoassay, coagulation, hematology, and others.
- By application, the market is divided into drug testing, infectious disease, nephrology, oncology, autoimmune disease, diabetes, cardiology, and others.
- By product, the market is classified into services, instruments, and reagents.
- By end-use, the market is bifurcated into home care, hospitals, laboratories, and others.
In-Vitro Diagnostics Market Regional Overview
The in-vitro diagnostics market is divided into five geographic segments: North America, Europe, Asia-Pacific, Latin America, and the MEA. As per the in-vitro diagnostics industry analysis, North America is currently the largest market for IVD and is expected to continue to dominate the market in the forecast period. This is due to a number of factors, including a large and aging population, a high prevalence of chronic diseases, and a strong healthcare infrastructure. In addition, the North American market has the benefits of a significant amount of major competitors in the market, cutting-edge technology, and high healthcare spending, all of which support its dominant position.
In-Vitro Diagnostics Market Players
Some of the major in-vitro diagnostics market companies are F. Hoffmann-La Roche AG, Abbott, bioMérieux SA, Danaher (Beckman Coulter, Inc.), Siemens, Sysmex, Bio-Rad Laboratories, Inc., QIAGEN, Thermo Fisher Scientific, and Becton, Dickinson and Company.
Click here to buy the Premium Market Research report https://www.acumenresearchandconsulting.com/buy-now/0/988
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/988
Mr. Frank Wilson
Acumen Research and Consulting
USA: +13474743864
India: +918983225533