Medical Device Testing Services Market Size to Reach USD 21.3 Billion by 2032 growing at 10.6% CAGR - Exclusive Report by Acumen Research and Consulting
The Medical Device Testing Services Market, valued at USD 8.6 Billion in 2023, is anticipated to surpass USD 21.3 Billion by 2032, reflecting a projected CAGR of 10.6%
The medical device testing services market is expanding rapidly, driven by the convergence of technology breakthroughs and increased regulatory requirements. The growing complexity of medical devices, fueled by advances in domains such as artificial intelligence, machine learning, and biotechnology, necessitates specialized testing services to verify their safety, efficacy, and regulatory compliance.
As a result, the need for medical device testing services is likely to rise in the future years, owing to the increasing usage of new technologies like 3D printing and nanotechnology, which are changing the way medical devices are designed, manufactured, and tested. The growing emphases on patient safety, as well as the necessity to comply with stringent regulatory standards imposed by the FDA, EU, and other global agencies, are all driving the expansion of the medical device testing services market.
Medical Device Testing Services Market Statistics
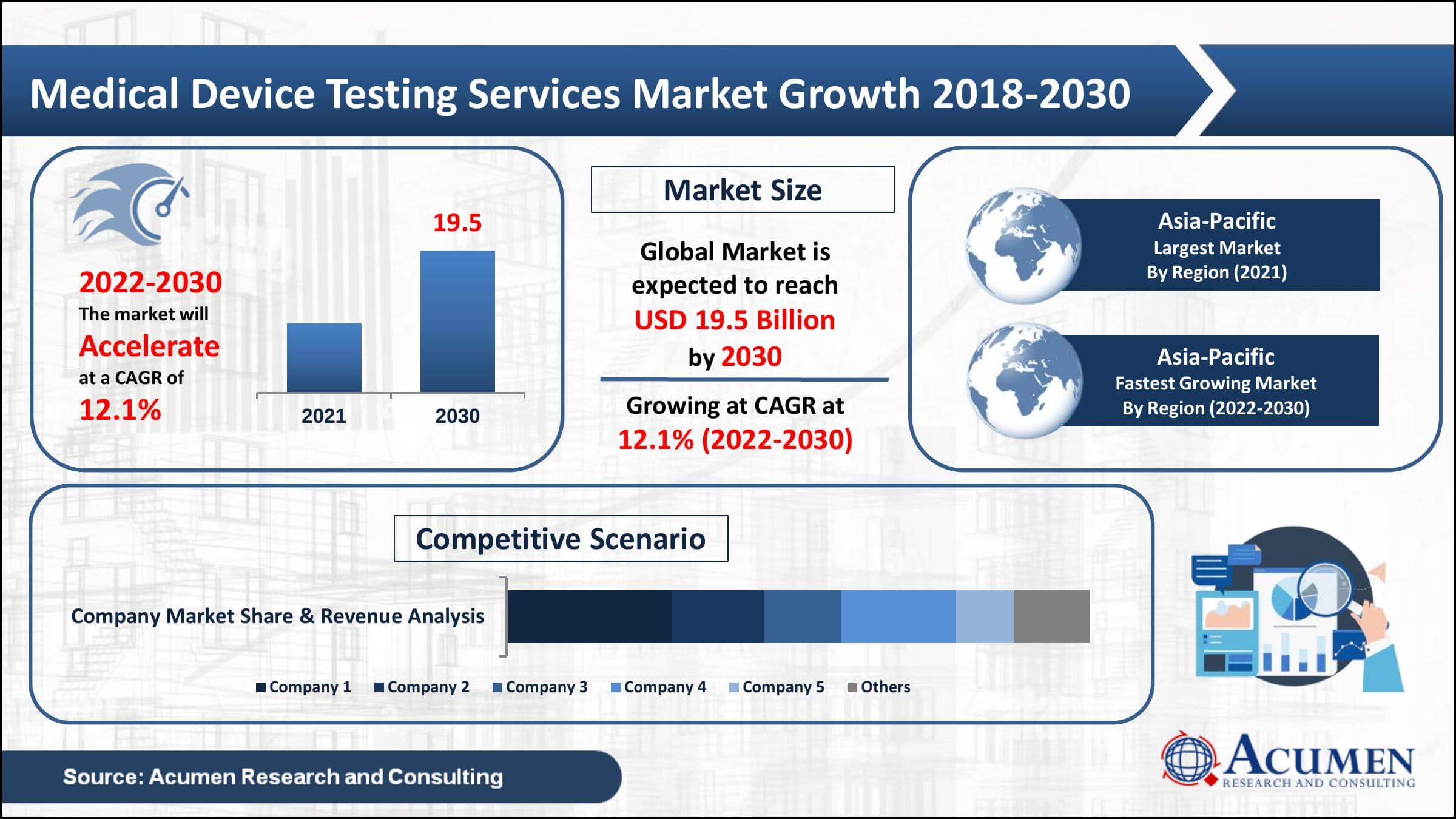
- In 2023, the global medical device testing services market revenue reached USD 8.6 billion
- The market is projected to grow at a CAGR of 10.6% from 2024 to 2032
- According to WHO, there are around 2 million different types of medical devices on the global market
- Asia-Pacific medical device testing services market share occupied around 41% in 2023
- By phases, the clinical capture over 70% of total market share in 2023
- Increasing focus on patient safety and compliance with strict regulatory requirements, propels the medical device testing services market size
Request for a sample of this premium research report@ https://www.acumenresearchandconsulting.com/request-sample/965
Medical Device Testing Services Market Dynamics
Medical device testing services are specialized services used to guarantee that medical gadgets are safe and functional. These services include, among other things, performance testing, biocompatibility testing, and sterilization validation. Medical device testing services also include assessing the suitability of materials utilized in the device, such as plastics, metals, and coatings, for use in a medical device. This comprises the chemical composition, mechanical qualities, and biocompatibility testing.
The medical device testing services market revenue is expected to grow significantly in the coming years due to the increasing demand for advanced and specialized testing services for medical devices. The growing complexity of medical devices and the need for specialized testing services, rising R&D spending in the medical device industry, and increased adoption of innovative technologies such as 3D printing and nanotechnology are likely to fuel market expansion. Furthermore, the growing emphasis on patient safety and the need to meet stringent regulatory criteria are driving the expansion of the medical device testing services market. Furthermore, with rising demand for advanced testing services such as biocompatibility testing and sterilization validation, there is a huge possibility for market expansion. The growing use of the Internet of Things (IoT) and wireless technology in medical equipment is also opening up new market prospects.
Advent of AI and IoT in Medical Device Testing Service Industry Offers Significant Market Opportunity
The introduction of Artificial Intelligence (AI) and the Internet of Things (IoT) technologies is altering the medical device testing services business, creating a substantial market opportunity. AI-powered testing platforms can scan complicated data sets to discover trends and abnormalities, allowing for more accurate and efficient medical device testing. Furthermore, IoT technology provides real-time monitoring of medical devices, resulting in speedier discovery and reporting of any problems or malfunctions. The convergence of AI and IoT is predicted to transform how medical devices are evaluated, allowing for more accurate, efficient, and cost-effective testing solutions.
The integration of AI and IoT into medical device testing services is predicted to have a significant impact on the industry, allowing for faster time-to-market, higher product quality, and lower costs. As the industry evolves, medical device makers will need specialized testing services that can use these technologies to assure the safety and efficacy of their products. The market opportunity for medical device testing services that can use the power of AI and IoT is enormous, as manufacturers attempt to capitalize on these technologies' benefits to stay ahead of the competition. As a result, the market is predicted to increase significantly in the next years, owing to the widespread implementation of AI and IoT in medical device testing services.
Medical Device Testing Services Market Segmentation
The global market for medical device testing services has been segmented into service, phase, and region
- Service segment is categorized into cardiovascular device's biocompatibility tests (orthopedic device's biocompatibility tests, dental implant devices' biocompatibility tests, dermal filler's biocompatibility tests, general surgery implantation devices biocompatibility tests, neurosurgical implantation devices biocompatibility tests, ophthalmic implantation device's biocompatibility tests, and others), chemistry test (analytical method development and validation, chemical characterization (E&L), and toxicological risk assessment and consulting), microbiology & sterility testing (bio-burden determination, pyrogen and endotoxin testing, sterility test and validation, anti-microbial activity testing, and other services), and package validation
- Phase segment is split into preclinical (antimicrobial wound dressings, medical coatings, and others) and clinical
- The medical device testing services market is geographically segmented across North America, Latin America, Europe, Asia-Pacific, and the Middle East and Africa
Medical Device Testing Services Market Regional Outlook
The global medical device testing services market is geographically segmented into Europe, Latin America, North America, Asia-Pacific, and the Middle East and Africa (MEA). According to industry analysis, North America is expected to grow with fastest rate, driven by the presence of numerous medical device manufacturers in the region and the increasing adoption of innovative technologies. The United States, in particular, is home to a significant number of medical device makers, many of which are global industry leaders.
The region's well-developed healthcare system also drives demand for advanced and specialized medical devices, which in turn fuels demand for specialized testing services. North America is at the forefront of technological advancements in the medical device industry, further driving demand for advanced and specialized testing services. The presence of renowned research institutions in the region also fosters innovation and development in the medical device industry, creating opportunities for medical device testing services providers.
Medical Device Testing Services Market Players
Medical device testing services companies profiled in the report include includes Sterigenics International LLC, Nelson Labs, American Preclinical Services LLC, Charles River Laboratories International, Inc., SGS SA, Eurofins Scientific, LLC, Intertek Group PLC, WuXiAppTec Group, North American Science Associates, Inc., Pace Analytical Services, LLC., and Toxikon, Inc.
Click here to buy the Premium Market Research report https://www.acumenresearchandconsulting.com/buy-now/0/965
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/965
Mr. Frank Wilson
Acumen Research and Consulting
USA: +13474743864
India: +918983225533