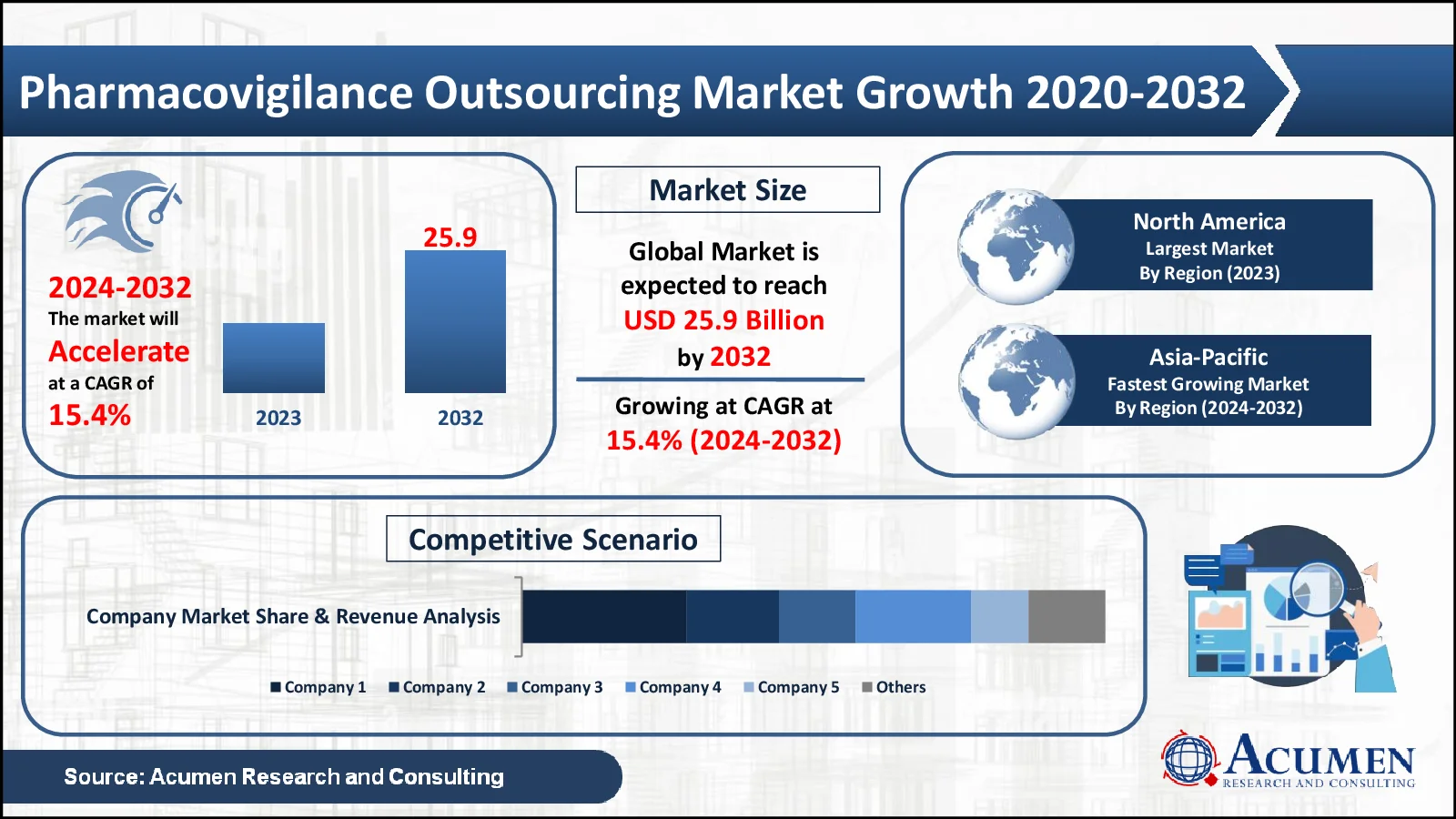
The Pharmacovigilance Outsourcing market, valued at USD 7.2 Billion in 2023, is projected to surpass USD 25.9 Billion by 2032, indicating a robust CAGR of 15.4%
The growing emphasis on new drug development for chronic disease treatments, remote monitoring for clinical operations, quality assurance, regulatory affairs, statistical analysis, and clinical trials, pharmaceutical companies preference for PV outsourcing facilities, and an increase in the number of adverse events and other safety concerns related to medical products have all contributed to the growth of the global pharmacovigilance outsourcing market.
The North American market is expected to grow at a significant CAGR due to the presence of a large number of major pharmaceutical and medical device businesses. Furthermore, high healthcare costs and growing concern about drug safety and side effects are driving regional market expansion.
Increasing usage of pharmacovigilance IT solutions and services, such as safety data integration and validation, design and development of safety data repositories, and deployment, is expected to drive market expansion over the forecast period. Pre-marketing services, on the other hand, are expected to grow significantly during the projected period as pharmaceutical companies increase their expenditure in the development of new pharmaceuticals. The growing demand for pre-marketing pharmacovigilance actions to obtain information about adverse medication effects from pre-clinical screening is driving the worldwide pharmacovigilance outsourcing market.
However, concerns over data security and confidentiality became a restraint for the pharmacovigilance outsourcing market. Moreover, integration of AI and machine learning in safety data analysis prompted the pharmacovigilance outsourcing market.
Pharmacovigilance Outsourcing Market Statistics
Access Table Of Content: https://www.acumenresearchandconsulting.com/table-of-content/pharmacovigilance-outsourcing-market
Pharmacovigilance Outsourcing Market Dynamics
Rising Volume of Adverse Drug Reactions (ADRs) Reporting Fuels the Pharmacovigilance Outsourcing Market Value
The rising number of adverse drug reactions (ADRs) reported by patients and healthcare professionals is driving the growth of the pharmacovigilance outsourcing industry. As more people experience side effects from medications, the need to closely monitor and manage these reactions has become more critical. To address this, pharmaceutical companies are increasingly outsourcing these responsibilities to specialized firms. These firms have the expertise to handle large volumes of data, ensuring that patient safety is prioritized while also meeting stringent regulatory requirements. This trend is boosting the demand for pharmacovigilance services, making them a key factor in the market’s expansion. Additionally, outsourcing allows companies to focus on core activities while relying on experts to manage complex pharmacovigilance tasks. This shift is contributing significantly to the growth and importance of pharmacovigilance in the healthcare industry.
Growing Demand for Personalized Medicine and Related Safety Monitoring Offer Significant Pharmacovigilance Outsourcing Market Opportunity
The increasing demand for personalized medicine, which customizes treatments for individual patients, is significantly boosting the need for specialized safety monitoring. As these therapies are highly complex and tailored, ensuring their safety is paramount. This creates a substantial opportunity in the pharmacovigilance outsourcing sector, where companies offer expert services to manage the safety of these individualized treatments. By outsourcing these critical tasks, pharmaceutical companies can concentrate on innovation while maintaining compliance with stringent safety standards. This trend is a key driver of growth in the pharmacovigilance outsourcing market, as it aligns with the industry's shift towards more personalized healthcare solutions.
Pharmacovigilance Outsourcing Market Segmentation
The global market for pharmacovigilance outsourcing has been segmented into service, service provider, and end-user, and region.
Pharmacovigilance Outsourcing Market Regional Outlook
In terms of pharmacovigilance outsourcing market analysis, North America is expected to be the top revenue-generating region in the global pharmacovigilance outsourcing market. This expansion is due to the presence of multiple big pharmaceutical and medical device businesses. Furthermore, many pharmaceutical companies are progressively outsourcing pharmacovigilance (PV) services to decrease operational expenses and total costs, which is fueling market growth.
The Asia-Pacific area is also predicted to increase significantly over the projection period, owing to China's strong pharmacovigilance system, which comprises organizations and legislation governing pharmacovigilance outsourcing. Regional market growth is heavily influenced by advancements in pharmaceutical production. Furthermore, strong and supportive policies aiming at improving the quality of pharmacovigilance services in India are expected to help drive regional market growth.
Pharmacovigilance Outsourcing Market Players
Pharmacovigilance outsourcing companies profiled in the report include Clintec, Covance, Oracle Corporation, iGATE Corporation, Ergomed, Accenture, IBM Corporation, iMED Global Corporation, Novartis, Bioclinica, Medpace Holdings, MarksMan Healthcare, SIRO Clinpharm, Symogen, IQVIA, and Parexel.
Enquire Before Buying https://www.acumenresearchandconsulting.com/inquiry-before-buying/1094
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/1094
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533