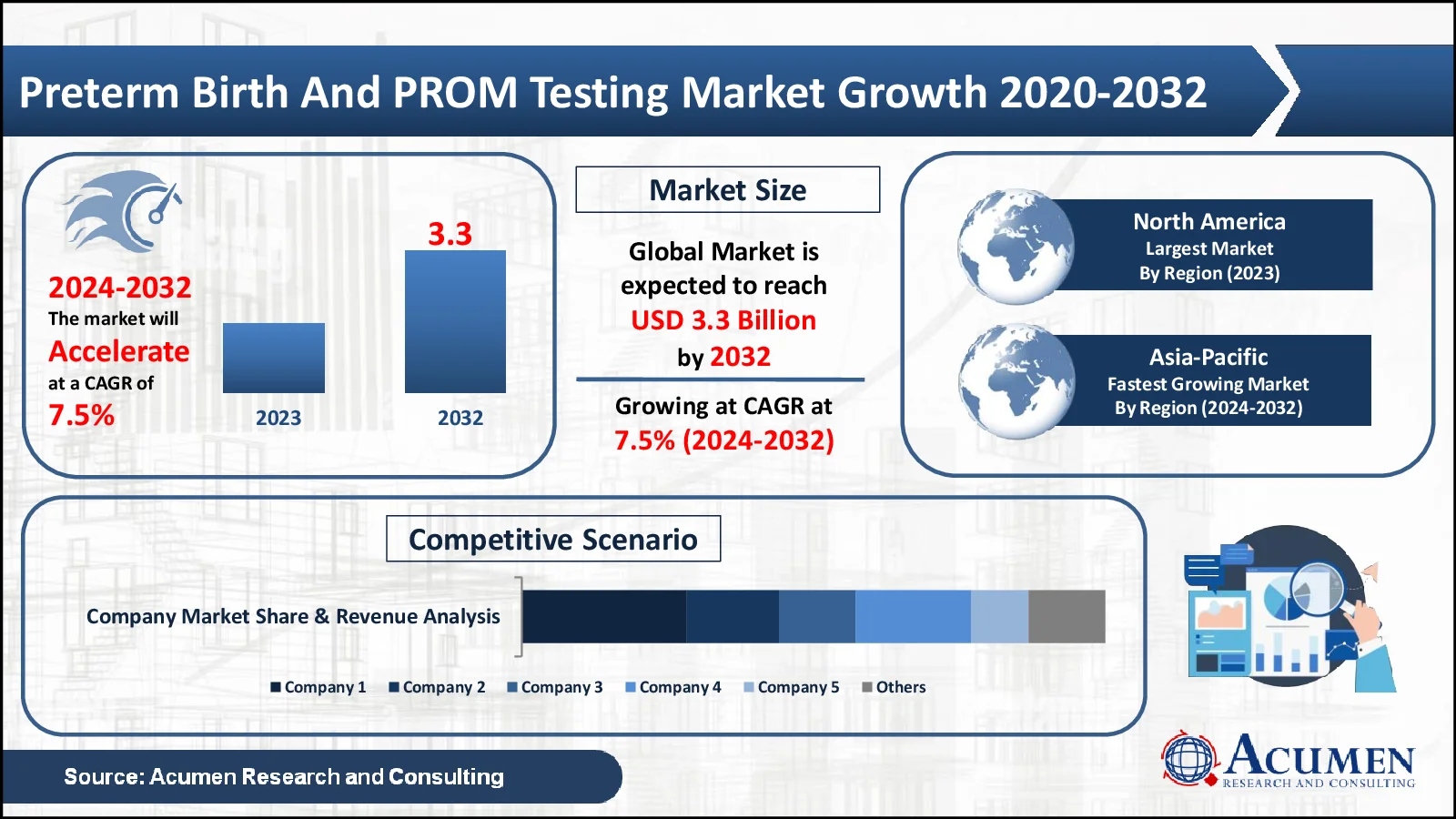
Preterm Birth And PROM Testing Market, valued at USD 1.7 Billion in 2023, is projected to surpass USD 3.3 Billion by 2032, indicating a robust CAGR of 7.5%
Preterm birth and PROM (Premature Rupture of Membranes) testing are crucial in monitoring pregnancies to avoid early labor and problems. Preterm birth diagnostics, such as ultrasounds and biomarker screenings, aid in the early detection of labor, whereas PROM testing, such as nitrazine or fetal fibronectin, reveal membrane rupture risks. Rising preterm birth rates, breakthroughs in diagnostic tools, and more mother health awareness are among the factors driving change. High testing costs, regulatory barriers, and limited availability in low-resource locations are some of the constraints. Moreover, expanding point-of-care testing, generating more reliable biomarkers, and meeting the growing demand for non-invasive, home-based diagnostic solutions which becomes significant opportunity for industry in forecast year.
However, high cost of advanced diagnostic tests became a restraint for preterm birth and PROM testing market. Furthermore, the rising average age of pregnant women (>35 years old) has increased the risk of health issues such as hypertension and reduced blood flow to the placenta. These complications endanger both the mother and the unborn, frequently resulting in premature deliveries or neonatal deaths. Despite the fact that traditional procedures, such as the Nitrazine and Ferning tests, can generate negative predictive value (NPV), they have relatively weak positive predictive values for unrestricted communication.
Preterm Birth And PROM Testing Market Statistics
Access Table Of Content: https://www.acumenresearchandconsulting.com/preterm-birth-and-prom-testing-market
Preterm Birth And PROM Testing Market Dynamics
Increasing Awareness of Maternal and Neonatal Health Fuels the Preterm Birth and Prom Testing Market Value
The growing awareness of maternal and newborn health is boosting the preterm birth and PROM testing industry. Governments, healthcare institutions, and non-governmental organizations (NGOs) are working to educate pregnant women about the dangers of preterm birth and the need of early detection. This increasing awareness has resulted in a growing demand for trustworthy diagnostic tools to monitor pregnant health, detect difficulties early on, and avert negative consequences. Furthermore, ads encouraging maternal and neonatal care encourage healthcare providers to use modern testing methods. As a result, the industry is on an upward trend, particularly in nations with expanding healthcare infrastructure.
Expansion of Point-Of-Care Testing In Remote Areas Offer Significant Preterm Birth and Prom Testing Market Opportunity
The growth of point-of-care (POC) testing in distant and underserved locations creates a substantial opportunity for the preterm birth and PROM testing market. POC testing allows for faster, on-site diagnoses, eliminating the need for hospital trips in areas with limited healthcare access. This is especially important for preterm birth and PROM, where early detection and intervention can help avoid significant consequences. The development of portable, user-friendly diagnostic gadgets enables healthcare providers to reach out to remote areas and provide more effective prenatal care. As global measures to promote maternal health continue, demand for point-of-care testing in these areas is likely to rise.
Preterm Birth And PROM Testing Market Segmentation
Preterm Birth And PROM Testing Market Regional Outlook
In terms of preterm birth and PROM testing market analysis, the North American market will account for the majority of worldwide market share. The rising occurrence of premature births, poor lifestyle choices, and increasing mother age are some of the reasons driving the market in this region. Healthcare infrastructure, broad use of breakthrough diagnostic technologies, and a high level of maternal and neonatal health awareness contributes to region’s strong growth in market. Strong government initiatives and research funding contribute to the development and implementation of preterm birth and PROM testing solutions. Furthermore, the existence of major market participants promotes growth in the region.
The Asia-Pacific market is expected to have the highest CAGR of 8.3% over the forecast period. Premature birth rates are high, and maternal health awareness is growing. Rising healthcare costs and ongoing government initiatives in developing economies bolster industry growth. The region's increasing access to new diagnostic technology contributes to its rapid expansion.
Preterm Birth And PROM Testing Market Players
Preterm Birth And PROM Testing companies profiled in the report include Abbott Laboratories, IQ Products, Hologic, Cooper Surgical, Biosynex, Qiagen, Medixbiochemica, Sera Prognostics, Coopersurgical Inc., and NX Prenatal; Inc.
Enquire Before Buying https://www.acumenresearchandconsulting.com/inquiry-before-buying/1263
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/1263
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533