November 2019
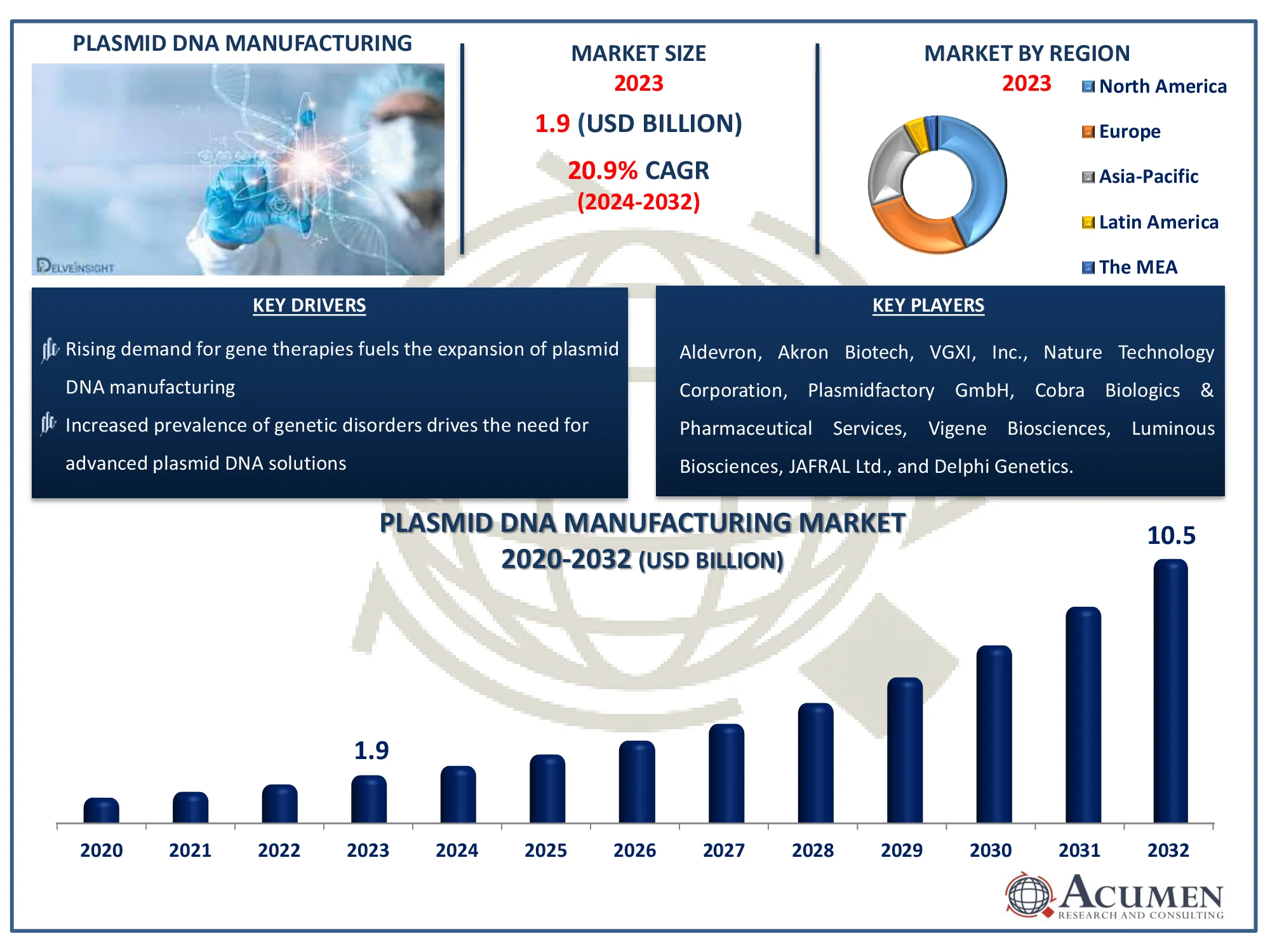
The Plasmid DNA Manufacturing Market, valued at USD 1.9 Billion in 2023, is set to expand to USD 10.5 Billion by 2032, growing at an impressive CAGR of 20.9%. Uncover key insights and future opportunities.
The Global Plasmid DNA Manufacturing Market Size accounted for USD 1.9 Billion in 2023 and is estimated to achieve a market size of USD 10.5 Billion by 2032 growing at a CAGR of 20.9% from 2024 to 2032.
Plasmid DNA Manufacturing Market Highlights
There are continuous development activities carried by major players in order to test plasmid DNA applicability. Usually plasmid DNA is prepared by cesium chloride density ultracentrifugation process and it has wide applications such as clones screening, sequencing, restriction digestion, cloning, and PCR. Plasmid DNA can be purified by various technique but most it is purified by using bacteria technique. These techniques include various methods such as alkaline lysis and ammonium acetate precipitation, and ion-exchange columns such as Qiagen columns, cesium chloride gradient separation, or PEG precipitation methods.
Global Plasmid DNA Manufacturing Market Dynamics
Market Drivers
Market Restraints
Market Opportunities
Plasmid DNA Manufacturing Market Report Coverage
| Market | Plasmid DNA Manufacturing Market |
| Plasmid DNA Manufacturing Market Size 2022 |
USD 8.6 Billion |
| Plasmid DNA Manufacturing Market Forecast 2032 | USD 10.5 Billion |
| Plasmid DNA Manufacturing Market CAGR During 2023 - 2032 | 20.9% |
| Plasmid DNA Manufacturing Market Analysis Period | 2020 - 2032 |
| Plasmid DNA Manufacturing Market Base Year |
2023 |
| Plasmid DNA Manufacturing Market Forecast Data | 2024 - 2032 |
| Segments Covered | By Grade, By Disease, By Development Phase, By Application, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Aldevron, Akron Biotech, VGXI, Inc., Nature Technology Corporation, Plasmidfactory GmbH, Cobra Biologics & Pharmaceutical Services, Vigene Biosciences, Luminous Biosciences, JAFRAL Ltd., and Delphi Genetics. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Plasmid DNA Manufacturing Market Insights
Increased cancer prevalence is expected to boost the production of plasmid DNA. In applying gene therapy to diagnose and treat diseases in patients, plasmid DNA is used. Increased awareness about gene therapy promotes the growth of plasmid DNA manufacturing market for the production of plasmid DNA during upcoming period. This is mainly due to increase in gene therapy products, accepted to treat rare diseases worldwide and availability of approved gene therapy products.
Plasmid DNA (pDNA) is the base for DNA vaccines and gene therapies for many infectious, acquired, and genetic diseases, including HIV-AIDS, Ebola, Malaria, and various cancer types, enteric pathogens, and influenza. The advantages of DNA vaccines are high, not contaminating and focusing on immune response only to those anti-immunization-wishing antigens and long-term persistence of immunization protection compared to conventional vaccines. These are the factors leading to the growth of plasmid DNA manufacturing. Well-developed infrastructure in developed countries that is able to support the R&D activities, high investment by government and private players, coupled with introduction of new various protocols in order to support the manufacturing of various products are some other major factors expected to support the growth of the plasmid DNA manufacturing market.
Gene therapy has various complications such as risk of mutagenesis, safety & efficacy issues and strict regulatory framework which is resulting in limited adoption thus hampering the growth of the market. Lack of developed infrastructure in order to conduct the R&D activities, limitations pertaining to investment for adoption of advanced technology, and low awareness of the treatment are among other major factors expected to hamper the growth of the target market. Developing nations have poor access to such sophisticated treatments and the costs of these are sky high making it out of reach to most of the population.
Technological progress to address traditional vector production challenges provides lucrative opportunities to the manufacturers. Development of an adaptive electroporation system for intratumoral plasmid DNA delivery where pro-inflammatory cytokine interleukin 12 encoded plasmid DNA intratumoral electroporation promotes innate and adaptive immune responses related to antitumor effects. Fixed parameters for pre-clinical tumors consisting of cells implanted into the skin are optimized in clinical electroporation conditions.
These conditions can be limited to clinically important tumors, because implanted models cannot detect the heterogeneity found in genetically engineered mouse models or clinical tumors. Variables affecting the result of treatment include tumor size, vascularization degree, fibrosis and necrosis that can produce the transfers of suboptimal genes and the results of variable therapies. To address this, an electroporation generator controlled by feedback was developed that can measure the electrochemical properties of tissue in real time.
Plasmid DNA Manufacturing Market Segmentation
The worldwide market for plasmid DNA manufacturing is split based on grade, disease, development phase, application, and geography.
Plasmid DNA Manufacturing Market By Grade
According to plasmid DNA manufacturing industry analysis, the GMP grade category dominates the market because of its importance in clinical and commercial biopharmaceutical applications. The GMP Grade category dominates the Plasmid DNA Manufacturing market because of its importance in clinical and commercial biopharmaceutical applications. This grade is required for sophisticated medicines such as CAR-T cell therapies and mRNA-based vaccinations, which explains its dominance. The growing global usage of gene-based medicines and vaccines drives up demand for GMP-grade plasmids. Furthermore, increased investments in R&D and clinical trials increase the demand for high-quality plasmids, establishing GMP-grade plasmid DNA as the market's preferred choice.
Plasmid DNA Manufacturing D Market By Disease
The cancer segment is the largest segment in the market and it is expected to witness significant growth over the plasmid DNA manufacturing market forecast period, owing to high usage of vectors for development of cancer therapies is the primary growth stimulant for the segment. In addition, constant research endeavors taken up to target cancer and gain approvals in various classes of biopharmaceutical drugs are supplementing the growth of the segment.
Plasmid DNA Manufacturing Market By Development Phase
The clinical therapeutics is the leading segment in the plasmid DNA manufacturing market due to the increased number of clinical studies concentrating on gene therapies, DNA vaccines, and cell-based medicines. Plasmid DNA is a critical component of these sophisticated treatments, delivering genetic material to target cells. The growing prevalence of genetic defects and infectious diseases has boosted the demand for plasmid DNA in therapeutic applications. Furthermore, regulatory agencies around the world have streamlined permission processes to encourage clinical research, which is driving the segment's growth. As pharmaceutical and biotech companies invest heavily in developing novel therapies, the need for plasmid DNA in clinical-stage pharmaceuticals develops, cementing its position as the market's leading category.
Plasmid DNA Manufacturing Market By Application
The cell & gene therapy sector has emerged as the major application in the plasmid DNA manufacturing market, owing to the increasing use of sophisticated therapeutic solutions for genetic disorders and chronic diseases. Plasmid DNA is important in gene editing technologies like CRISPR-Cas9, as well as in the development of viral vectors for gene delivery. The expanding prevalence of genetic disorders, together with increased financing for cell and gene therapy research, is driving this segment's expansion. Furthermore, its applications in regenerative medicine and individualized treatment techniques reinforce its position in the growing market landscape.
Plasmid DNA Manufacturing Market Regional Outlook
North America
Europe
Asia-Pacific
Latin America
The Middle East & Africa
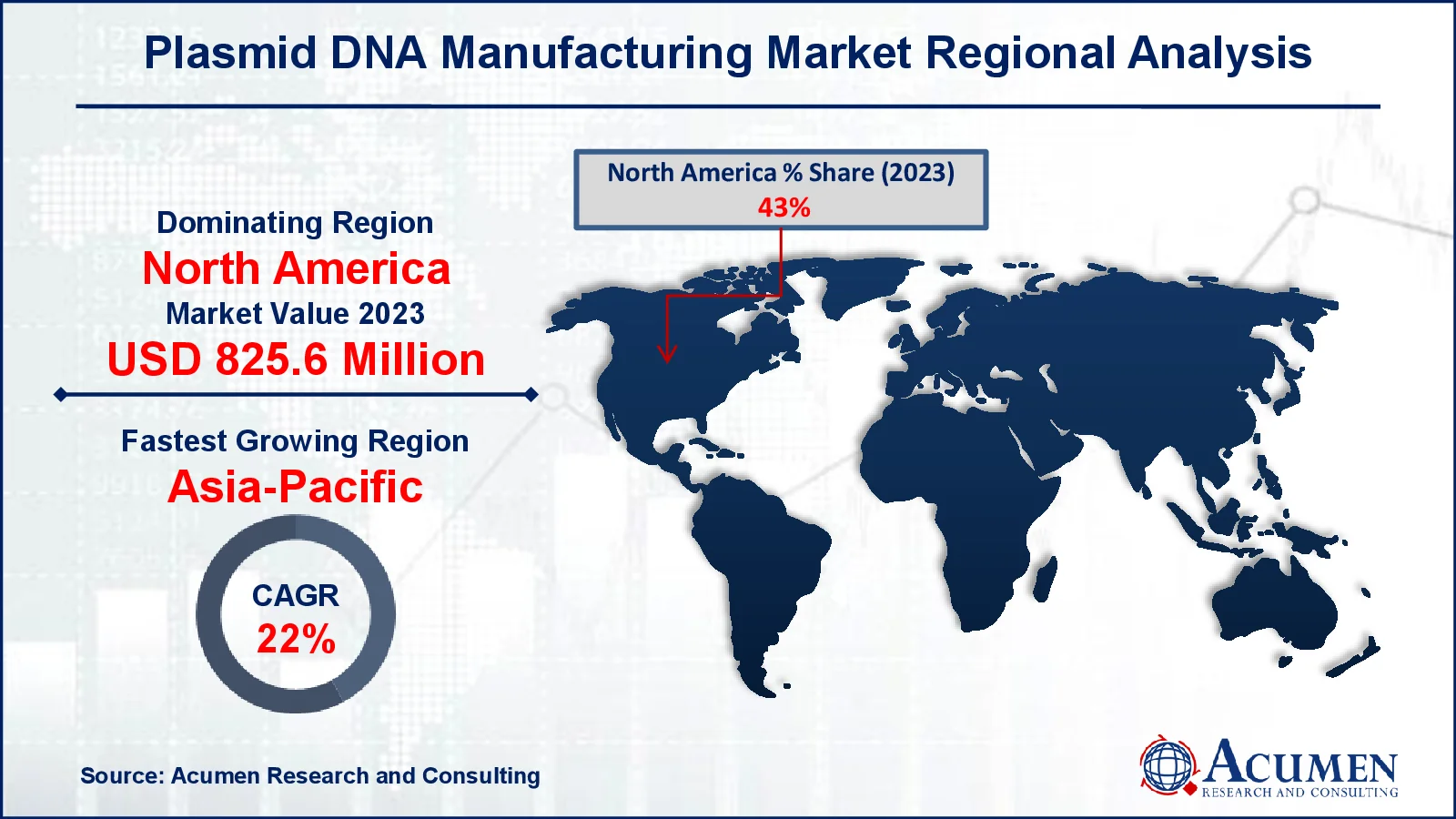
Plasmid DNA Manufacturing Market Regional Analysis
The North America plasmid DNA manufacturing market size was over 825.6 million USD in 2023 and is expected to witness the growth at a notable from 2024 to 2032. North America is expected to dominate in the global plasmid DNA manufacturing market. This can be attributed to the presence of several biopharmaceutical manufacturers in the U.S., coupled with a strong and effective regulatory structure for development of advanced therapies in the country. Besides this, recent approval of gene therapies by U.S. FDA is projected to augment the demand for plasmid DNA manufacturing market in the coming years.
The market in Europe is expected to contribute significant revenue share in the global market, owing to significant incentives offer through Orphan Medicinal Products Regulations (European Union) that has encouraged pharmaceutical and biotechnology companies to consider the development of rare disease medicines as a potentially profitable endeavor.
The market in Asia Pacific is expected to witness faster growth, owing to factors such as increasing government expenditure on healthcare sector in order to facilitate R&D activities. In addition, growing government initiatives and increased investments by private companies related to R&D activities are likely to fuel this regional market.
Plasmid DNA Manufacturing Market Players
Some of the top plasmid DNA manufacturing market companies offered in our report includes Aldevron, Akron Biotech, VGXI, Inc., Nature Technology Corporation, Plasmidfactory GmbH, Cobra Biologics & Pharmaceutical Services, Vigene Biosciences, Luminous Biosciences, JAFRAL Ltd., and Delphi Genetics.
Looking for discounts, bulk pricing, or custom solutions? Contact us today at sales@acumenresearchandconsulting.com
November 2019
December 2024
December 2024
October 2022