April 2021
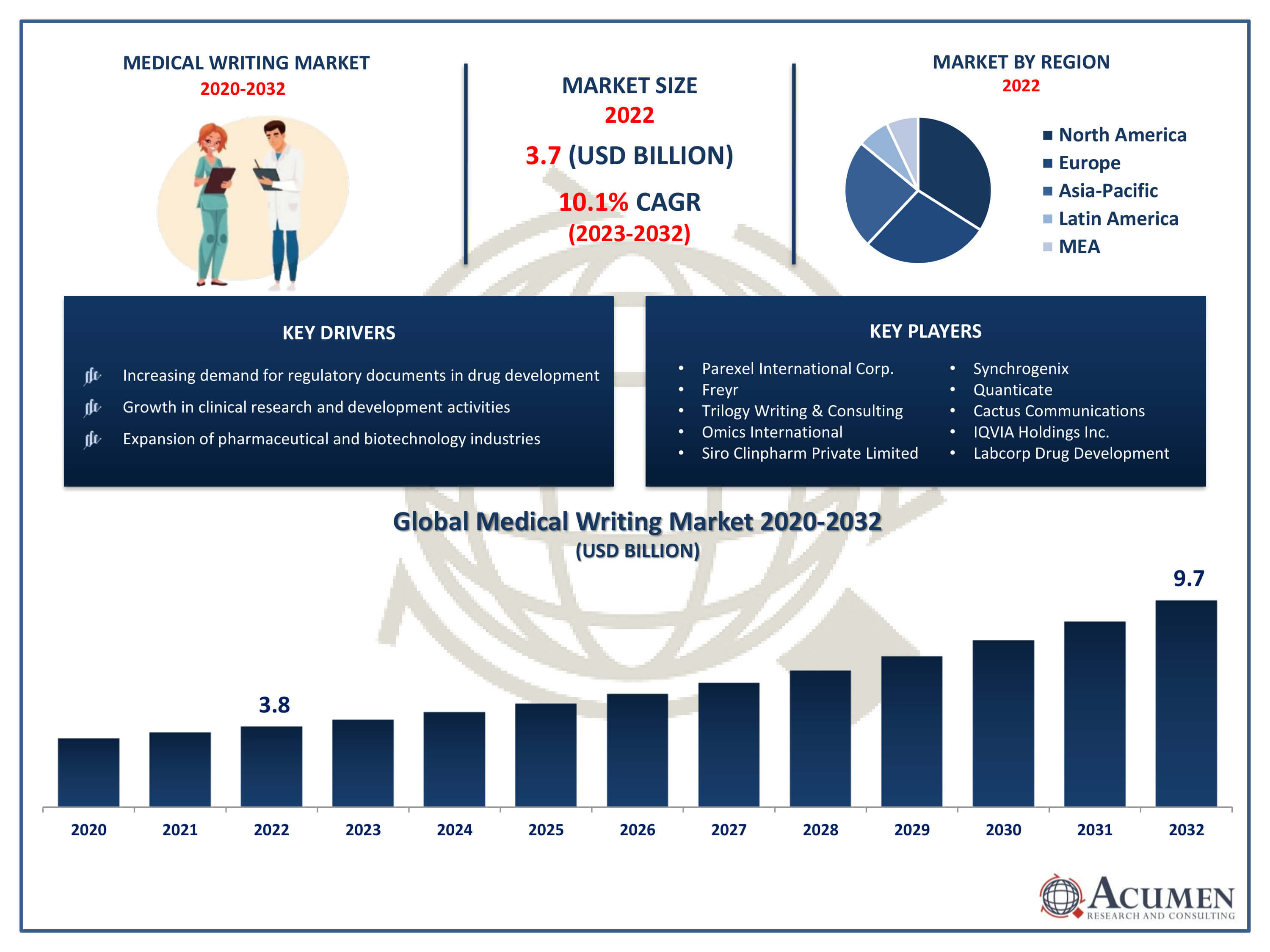
Medical Writing Market Size accounted for USD 3.8 Billion in 2022 and is projected to achieve a market size of USD 9.7 Billion by 2032 growing at a CAGR of 10.1% from 2023 to 2032.
The Medical Writing Market Size accounted for USD 3.8 Billion in 2022 and is projected to achieve a market size of USD 9.7 Billion by 2032 growing at a CAGR of 10.1% from 2023 to 2032.
Medical Writing Market Highlights
Medical writing is a specialized form of technical writing that involves creating documents and content related to healthcare, medicine, and life sciences. It encompasses a wide range of materials, including regulatory documents for drug approval, clinical trial protocols and reports, scientific manuscripts for publication in journals, educational materials for healthcare professionals and patients, marketing materials for pharmaceutical companies, and more. Medical writers must have a strong understanding of scientific concepts and research methodologies, as well as excellent writing and communication skills to effectively convey complex information to various audiences.
The market for medical writing has experienced significant growth in recent years due to several factors. One major driver is the increasing demand for new drugs and medical devices, which has led to a rise in clinical research and development activities. As a result, there is a growing need for regulatory documents and publications to support these endeavors. Additionally, the expanding pharmaceutical and biotechnology industries, along with advancements in medical technology, have created opportunities for medical writers to contribute to a wide range of projects. Furthermore, the globalization of clinical trials and the requirement for documents to comply with international regulatory standards have further fueled the demand for skilled medical writers.
Global Medical Writing Market Trends
Market Drivers
Market Restraints
Market Opportunities
Medical Writing Market Report Coverage
| Market | Medical Writing Market |
| Medical Writing Market Size 2022 | USD 3.8 Billion |
| Medical Writing Market Forecast 2032 | USD 9.7 Billion |
| Medical Writing Market CAGR During 2023 - 2032 | 10.1% |
| Medical Writing Market Analysis Period | 2020 - 2032 |
| Medical Writing Market Base Year |
2022 |
| Medical Writing Market Forecast Data | 2023 - 2032 |
| Segments Covered | By Type, By Application, By End Use, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Parexel International Corporation, Freyr, Trilogy Writing & Consulting GmBH, Omics International, Siro Clinpharm Private Limited, Synchrogenix, Quanticate, Cactus Communications, IQVIA Holdings Inc., Labcorp Drug Development, and Inclin, Inc. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Medical writing is a specialized field that involves creating a wide range of documents and content related to healthcare, medicine, and life sciences. These documents serve various purposes within the medical and scientific community, including regulatory submissions, clinical trial protocols and reports, scientific publications, educational materials, marketing collateral, and more. Medical writers play a crucial role in translating complex scientific information into clear, concise, and accurate content that is accessible to different audiences, including healthcare professionals, regulatory authorities, patients, and the general public. The applications of medical writing are diverse and encompass many areas within the healthcare industry. In clinical research, medical writers are involved in drafting protocols, informed consent forms, and clinical study reports, which are essential for conducting and documenting the results of clinical trials.
The medical writing market has witnessed substantial growth in recent years, driven by various factors. One significant contributor to this expansion is the increasing complexity of regulatory requirements in the pharmaceutical and healthcare industries. As drug development processes become more rigorous and stringent, the demand for specialized regulatory documents, such as clinical trial protocols, investigator brochures, and regulatory submissions, has surged. Moreover, the globalization of clinical trials has necessitated the production of documents that comply with international regulatory standards, further fueling the need for skilled medical writers who can navigate these complex requirements. Additionally, the growing emphasis on evidence-based medicine and the dissemination of scientific research has led to an increased demand for scientific publications and medical communications. Medical writers play a crucial role in translating clinical trial data and research findings into clear and concise manuscripts for publication in peer-reviewed journals, as well as creating educational materials for healthcare professionals and patients.
Medical Writing Market Segmentation
The global Medical Writing Market segmentation is based on type, application, end use, and geography.
Medical Writing Market By Type
According to the medical writing industry analysis, the clinical writing segment accounted for the largest market share in 2022. Clinical writing encompasses a variety of documents critical to the development and approval of pharmaceuticals and medical devices. This segment includes the creation of clinical trial protocols, clinical study reports, informed consent forms, patient narratives, and other regulatory documents required for drug submissions to regulatory authorities such as the FDA and EMA. As the pharmaceutical industry continues to innovate and develop new therapies, the demand for clinical writing services has expanded correspondingly. One key driver of growth in the clinical writing segment is the increasing complexity and volume of clinical trials being conducted globally. As drug development pipelines expand and clinical research becomes more complex, there is a greater need for comprehensive and compliant documentation throughout the trial process. Additionally, the globalization of clinical trials has led to a rise in the number of multinational studies, further driving demand for clinical writing services to ensure consistency and adherence to regulatory standards across different regions.
Medical Writing Market By Application
In terms of applications, the medical journalism segment is expected to witness significant growth in the coming years. One significant catalyst is the increasing demand for accurate and timely health information among the general public. With rising health awareness and concerns, individuals are seeking reliable sources of medical news and insights to stay informed about advancements in healthcare, disease prevention, and treatment options. This has led to a surge in the consumption of medical journalism across various platforms, including print, online publications, social media, and podcasts. Moreover, the COVID-19 pandemic has further underscored the importance of medical journalism in disseminating critical information to the public. As the pandemic unfolded, there was a heightened need for clear and accessible reporting on topics such as transmission dynamics, public health measures, vaccine development, and emerging variants. Medical journalists played a crucial role in translating complex scientific data and expert opinions into digestible formats for diverse audiences, helping to combat misinformation and promote public health literacy.
Medical Writing Market By End Use
According to the medical writing market forecast, the contract research organizations (CROs) segment is expected to witness significant growth in the coming years. CROs provide a range of services to pharmaceutical, biotechnology, and medical device companies, including clinical trial management, regulatory affairs, and medical writing. As pharmaceutical companies increasingly outsource various aspects of drug development to CROs to streamline operations and reduce costs, the demand for medical writing services offered by these organizations has expanded significantly. CROs leverage their expertise and infrastructure to deliver high-quality medical writing solutions tailored to the specific needs of their clients, including the preparation of regulatory documents, clinical trial protocols, and scientific manuscripts. One key factor fueling the growth of the CROs segment in the medical writing market is the globalization of clinical trials. As drug development activities become increasingly globalized, pharmaceutical companies rely on CROs with global reach and local expertise to navigate regulatory requirements in different regions and countries.
Medical Writing Market Regional Outlook
North America
Europe
Asia-Pacific
Latin America
The Middle East & Africa
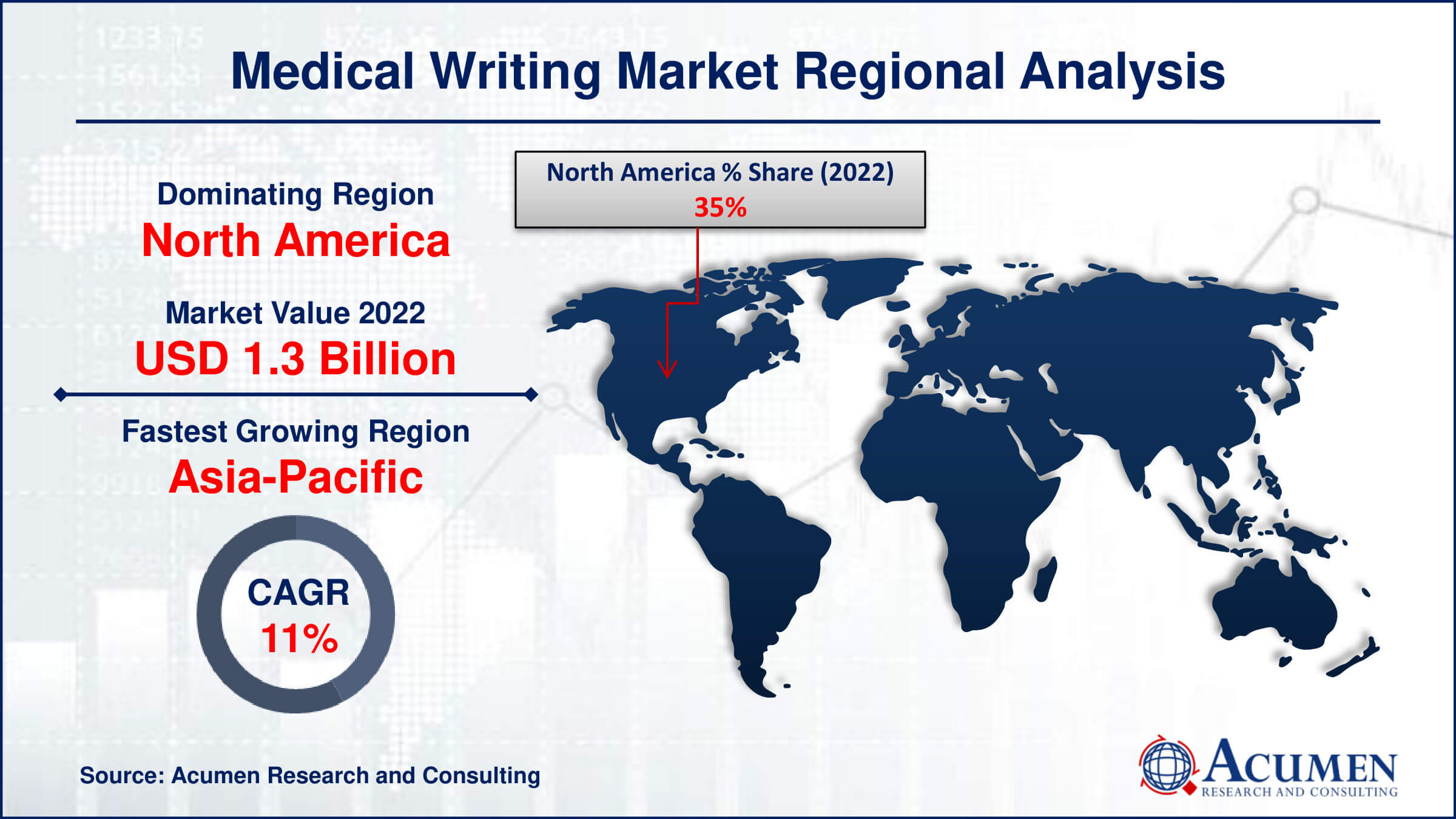
Medical Writing Market Regional Analysis
North America dominates the medical writing market for several reasons, primarily due to its robust pharmaceutical and biotechnology industries, advanced healthcare infrastructure, and regulatory environment. The region is home to a significant number of pharmaceutical companies, biotechnology firms, and contract research organizations (CROs) that drive demand for medical writing services. These organizations conduct extensive clinical research and development activities, necessitating the production of a wide range of regulatory documents, clinical trial protocols, and scientific publications. Additionally, North America boasts a highly skilled workforce, including medical writers with expertise in diverse therapeutic areas, scientific disciplines, and regulatory requirements, further strengthening its position as a leader in the medical writing market. Furthermore, North America benefits from a favorable regulatory landscape that fosters innovation and supports the timely approval of new drugs and medical devices. Regulatory agencies such as the Food and Drug Administration (FDA) in the United States and Health Canada play a pivotal role in ensuring the safety, efficacy, and quality of healthcare products, driving the demand for regulatory writing services. Medical writers in North America are well-versed in navigating these regulatory frameworks and have extensive experience in preparing documentation for regulatory submissions, compliance audits, and inspections.
Medical Writing Market Player
Some of the top medical writing market companies offered in the professional report include Parexel International Corporation, Freyr, Trilogy Writing & Consulting GmBH, Omics International, Siro Clinpharm Private Limited, Synchrogenix, Quanticate, Cactus Communications, IQVIA Holdings Inc., Labcorp Drug Development, and Inclin, Inc.
Looking for discounts, bulk pricing, or custom solutions? Contact us today at sales@acumenresearchandconsulting.com
April 2021
November 2024
December 2020
July 2022