April 2021
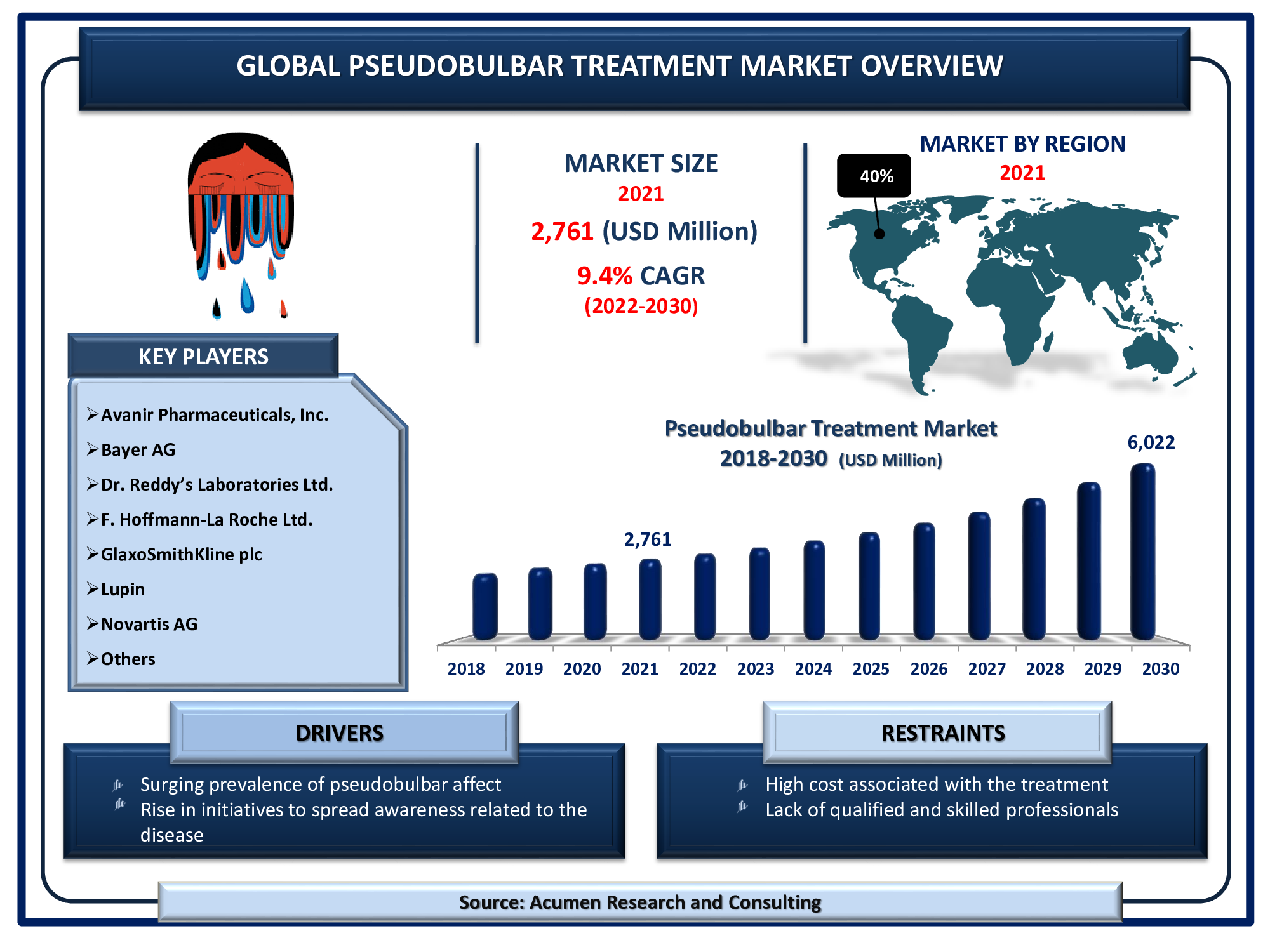
Pseudobulbar Treatment Global Market will achieve a market size of USD 6,022 Million by 2030, budding at a CAGR of 9.4%
The Global Pseudobulbar Treatment Market Size accounted for USD 2,761 Million in 2021 and will achieve a market size of USD 6,022 Million by 2030, budding at a CAGR of 9.4%.
Pseudobulbar affect (PBA) is a condition that's characterized by episodes of sudden uncontrollable and inappropriate laughing or crying. Pseudobulbar affect typically occurs in people with certain neurological conditions or injuries, which might affect the way the brain controls emotion. Increasing prevalence of pseudobulbar affect is the primary factor boosting the global pseudobulbar treatment market revenue. Furthermore, the increasing number of research and development activities is expected to be a prominent pseudobulbar treatment market trend fueling industry growth from 2022 to 2030.
Global Pseudobulbar Treatment Market DROs:
Drivers
Restraints
Opportunities
Report Coverage
| Market | Pseudobulbar Treatment Market |
| Market Size 2021 | USD 2,761 Million |
| Market Forecast 2030 | USD 6,022 Million |
| CAGR During 2022 - 2030 | 9.4% |
| Analysis Period | 2018 - 2030 |
| Base Year | 2021 |
| Forecast Data | 2022 - 2030 |
| Segments Covered | By Drugs, By Treatment, By Route of Administration, By End-Users, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Avanir Pharmaceuticals, Inc., Bayer AG, Dr. Reddy’s Laboratories Ltd., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Lupin, Mylan N.V., Novartis AG, Pfizer Inc., Sanofi, and Teva Pharmaceutical Industries Ltd. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Regulation Analysis |
| Customization Scope |
10 hrs of free customization and expert consultation |
PBA is affecting a large number of people across the globe. Pseudobulbar affect (PBA) is a condition that's characterized by episodes of sudden uncontrollable and inappropriate laughing or crying. It is witnessed among patients suffering from multiple sclerosis (MS), traumatic brain injury, and Alzheimer's disease. According to CDC, in 2020, as many as 5.8 million Americans were living with Alzheimer’s disease. In 2017, around 2 million people in the U.S. were suffering from pseudobulbar affect. Pseudobulbar affect is primarily associated with neurological disorders, and around 30% to 35% of patients suffer from depression. Prevalence of pseudobulbar affect is higher in people with neurological disorders, which is around 5% to 50%. A rapid increase in the number of patients suffering from PBA and increasing awareness among consumers related to the availability of advanced treatments are factors expected to drive the growth of the global pseudobulbar treatment market. Increasing R&D activities by major players rise in clinical trial activities for the development of new drugs, and approvals from the regulatory bodies are factors expected to segment the growth of the pseudobulbar treatment market.
Favorable business policies by the government and the emergence of small & Mid-size enterprises with innovative solutions are factors expected to boost the target market growth. Factors such as high costs associated with R&D activities of drugs and strict government regulations related to product approval are expected to hamper the growth of the global pseudobulbar treatment market. In addition, the lack of developed infrastructure in developing countries for R&D activities is expected to challenge the growth of the target market. However, huge investment by manufacturers for drug discovery and inclination towards tracking the untapped market in developing regions are factors expected to create new opportunities for players operating in the pseudobulbar treatment market over the forecast period. In addition, the government of developing countries focus on strengthening the regional manufacturing capabilities is expected to attract players further responsible for the revenue growth of the market.
Pseudobulbar Treatment Market Segmentation
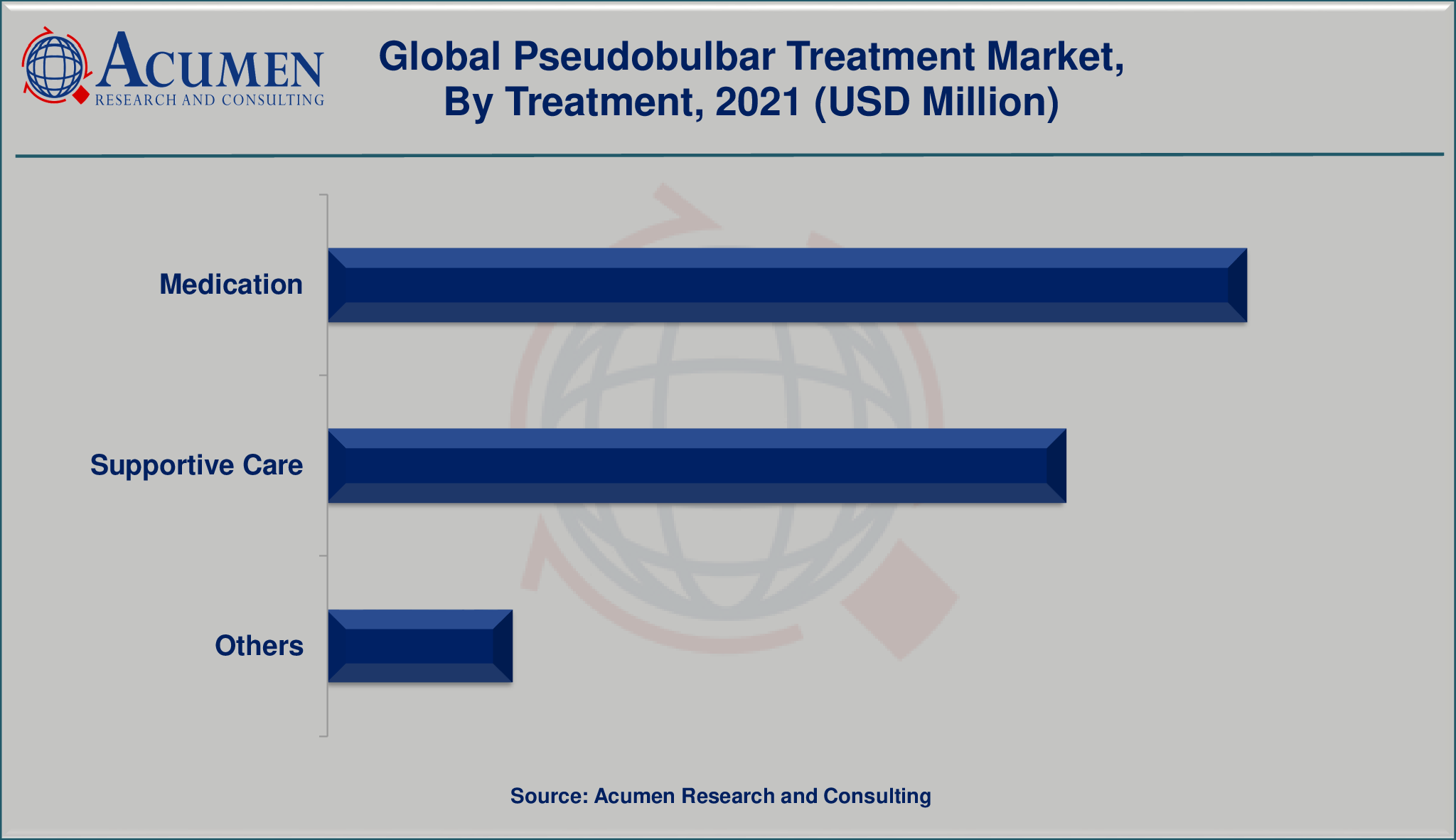
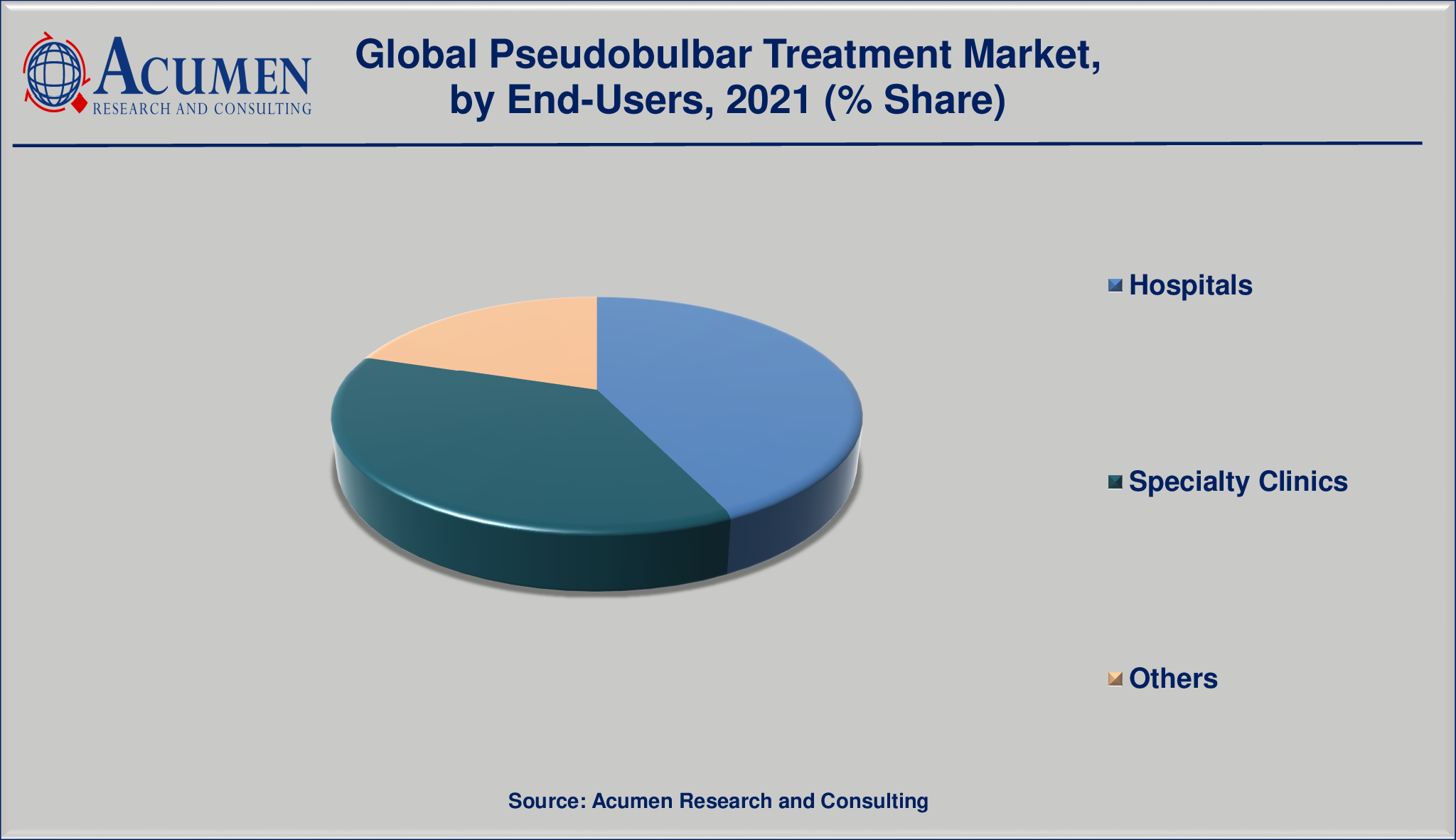
The global pseudobulbar treatment market is segmented into treatment, drugs, route of administration, and end-user. The treatment segment is divided into medication, supportive care, and others. The drug segment is bifurcated into pseudobulbar treatments, tricyclic antidepressants, nuedexta, and others. Among drugs, the tricyclic antidepressants segment is expected to witness faster growth in the target market.
Market by Drugs
Market by Treatment
Market By Route of Administration
Market By End-Users
Pseudobulbar Treatment Market Regional Outlook
North America
Europe
Latin America
Asia-Pacific
The Middle East & Africa (MEA)
Presence of high neurological disorders patients in North America region, fuels the regional market growth
The market in North America is expected to account for a major revenue share in the global pseudobulbar treatment market due to the high patient pool suffering from neurological disorders. High investment by the government and a collaborative approach between public-private players for product development are factors expected to support the growth of the pseudobulbar treatment market. In addition, the presence of the large number of players operating in the country and increasing partnership activities for business expansion are factors expected to support the regional market growth.
Pseudobulbar Treatment Market Players
Some of the top pseudobulbar treatment companies offered in the professional report include Avanir Pharmaceuticals, Inc., Bayer AG, Dr. Reddy’s Laboratories Ltd., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Lupin, Mylan N.V., Novartis AG, Pfizer Inc., Sanofi, and Teva Pharmaceutical Industries Ltd.
The global pseudobulbar treatment market is highly competitive due to the presence of a large number of players and innovative product offerings. In addition, business expansion activities through partnerships and agreements are factors expected to further increase the competition.
Players are focused on enhancing the business through the introduction of new solutions this is expected to attract new customers.
In 2020, Janssen Pharmaceutical Companies, a global drug manufacturer received approval from the U.S. Food and Drug Administration (FDA) for “SPRAVATO”. SPRAVATO is the first and only approved medicine that has been shown to reduce depressive symptoms within 24 hours, providing a new option for significant symptom relief until a longer-term, comprehensive treatment plan can take effect.
In 2018, Avanir pharmaceuticals, a global drug manufacturer launched a national, multi-channel campaign aimed at raising awareness of Pseudobulbar Affect, an often ignored and under-treated neurologic condition that occurs secondary to certain neurologic injury or disease.
Looking for discounts, bulk pricing, or custom solutions? Contact us today at sales@acumenresearchandconsulting.com
April 2021
June 2022
February 2024
February 2025